back...
By furnishing the polymers with additives, the mechanical, electrical and chemical properties are improved, the processing is eased and the appearance of the product is changed.
The mechanical properties are changed by
* fillers
* nucleation agents
* softening agents
* foaming agents
The chemical properties are influenced by
* UV agents
* thermo stabilizers
* flame retardants
The appearance can be changed by
* pigments
* dies
* optical brighteners
The surface properties can be changed by
* antistatic agents
* slip agents
* antiblock agents
Additives can be used by themselves, as a combination of additives or as so called batches or masterbatches which contain a carrier resin.
A masterbatch, therefore, represents a concentrate of an additive or a combination of additives in a polymer.
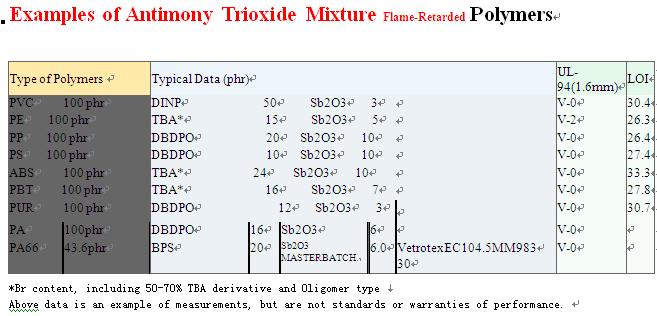
We can supply any quantity and any kind of Antimony products and fire retardant from stock.would you please inform us how many you need and your target price, then we will confirm ASAP. We are sincerely hope to do business with you and establish long term business relationship with your respectable company.
Look forward to hearing from you soon.
Best regards,
Sam Xu
MSN: xubiao_1996@hotmail.com
GMAIL: samjiefu@gmail.com
SKPYE:jiefu1996
Fire retardant masterbatch
...
read more