
Antimony
Antimony is the fourth element in Group 15 of the periodic table. Its atomic number is 51, its atomic mass is 121.75, and its chemical symbol is Sb.
Properties
Antimony is a metalloid. It exists in three allotropic forms: a silvery white metal; a yellow, crystalline solid; and an amorphous black powder. Its melting point is 1,166°F (630°C) and its boiling point is 2,975°F (1,635°C). Its most common allotropic form, the silver- white metal, is a relatively soft material that can be scratched by glass. Its density is 6.68 grams per cubic centimeter.
Occurrence and Extraction
Antimony is rarely found in nature as an element. Its most common ore is the mineral stibnite, a form of antimony sulfide (Sb2S3). Pure antimony can be obtained from antimony sulfide by heating the compound with hot iron: 2Fe + Sb2S3 Fe2S3 + 2Sb.
The usual source of most antimony produced today is the recycling of metal alloys. About half of the antimony produced in the United States is recycled from old lead storage batteries, in which the antimony was originally alloyed with lead.
Discovery and Naming
Compounds of antimony have been known and used by humans for centuries. Probably the first person to describe the element in detail was the French chemist Nicolas Lemery (1645-1715). The origin of the element's name is uncertain, but probably comes from two Arabic words anti and monos that mean "not alone." The name was chosen because antimony does not occur alone in nature, only in compounds.
Uses
Antimony is usually used in the form of an alloy. Lead- antimony alloys were once very widely used for solder, ammunition, fishing tackle, covering for electrical cables, low- melting alloys, and batteries. Such uses are now decreasing because of the serious health problems posed by lead. Antimony and its compounds are also used in transistors, the manufacture of ceramics and glass, and the production of plastic.
World of Scientific Discovery© on Antimony
Antimony
Antimony is a metal element with the atomic number of 51. Its chemical symbol, Sb, is taken from the Latin name for the element, stibnium, or stibium. The name is meant to suggest "a metal that does not occur by itself."
Antimony has a bluish-white metallic luster and an atomic weight of 121.757. It is very brittle and has a flaky texture. Its melting point is 1,167°F (630.6°C) and its boiling point, 2,888.6°F (1,587°C). Chemically, antimony is a metalloid, or semi-metal. That is, it may behave either as a metal or as a non- metal, depending on the chemical environment in which it exists.
Compounds of antimony have been used by humans throughout history. The Bible describes--and condemns--the use by some women of a " stibic stone" to paint their faces. The stibic stone was probably made of antimony (III) sulfide, Sb2S3, a naturally occurring black mineral. Women in many cultures used the material as an eye-liner to make their eyes appear larger and more striking.
Antimony was also used in coloring glass and making vases in the pre-Christian era. Craftspeople knew nothing about antimony as an individual element and classified it, along with most metals, as "lead."
Alchemists of the Middle Ages frequently wrote about a substance they called "antimony." The substance was, in fact, probably some form of antimony (III) sulfide. Until 1771, there was a good deal of confusion as to exactly what the name antimony referred to--the element, one of its compounds, or both. In that year, Jean-Baptiste Buquet suggested that the name be reserved for the metal.
The free element is obtained from the naturally occurring sulfide by roasting (to produce the oxide) and then reduction with scrap iron or carbon. Today, about half of the antimony in the United States is obtained by recycling the metal from lead storage batteries.
Scientists have known for more than 200 years that antimony can strengthen or change the physical properties of metals with which it is alloyed. An early eighteenth-century writer, Geoffrey the Elder, described the use of antimony in making bells, tools, and type for printing presses. Even today, about half of all the antimony produced is used in alloys for uses such as these.
For example, antimony is added to the lead used in lead storage batteries to make the lead harder and stronger. Alloys containing up to 20 percent antimony are also used in making type metal, bullets, and cable sheathing. Small but increasingly important quantities of antimony are used as impurities in the manufacture of semiconductors, especially those used in infrared detectors.
The rest of the antimony produced today is used to make compounds such as the oxides and sulfides, Sb2O3 and Sb 2S3, sodium antimonate, NaSbO3, and antimony (III) chloride, SbCl3. These compounds, in turn are used in the manufacture of paints, matches, ceramics, fireworks, percussion caps, and pharmaceuticals. They are also used in the dyeing of cloth and in the fire-proofing of materials. Although one compound, tartar emetic (antimony potassium tartrate) has long been used as a medicine, most compounds of antimony are toxic.
Chemical Elements© on Antimony
Antimony
Symbol
Sb
Atomic Number
51
Atomic Mass
121.75
Family
Group 15 (VA) Nitrogen
Pronunciation
AN-ti-moh-nee
Overview
Antimony compounds have been used by humans for centuries. Women of ancient Egypt used stibic stone, antimony sulfide, (Sb2S3), to darken their eyes. Antimony was also used in making colored glazes for beads and glassware. The chemical symbol for antimony was taken from the ancient name for the element, stibium. Not recognized as a chemical element until the Middle Ages, antimony became a common material used by alchemists.
Alchemy was a kind of pre-science that existed from about 500 B.C. to about the end of the 16th century. Alchemists wanted to find a way of changing lead, iron, and other metals into gold. They also wanted to find a way of having eternal life. Alchemy contained too much magic and mysticism to be a real science, but alchemists developed a number of techniques and produced many new materials that were later found to be useful in modern chemistry. Antimony was one of these materials.
Antimony is a metalloid. A metalloid is an element that has characteristics of both metals and non-metals. The metalloids can be found on either side of the staircase line on the right side of the periodic table (with the exception of aluminum, which is not considered a metalloid).
Antimony is primarily used in alloys, ceramics and glass, plastics, and flame retardant materials. Flame retardant materials do not burn with an open flame. Instead, they smolder or do not bum at all.
Discovery and Naming
Compounds of antimony were known to ancient cultures. They have been found, for example, in the colored glazes used on beads, vases, and other glassware. But these compounds were not widely used until the Middle Ages when they became popular among alchemists. They thought that antimony could be used to convert lead into gold. It was during this period that records about the properties of antimony begin to appear.
The element was probably first named by Roman scholar Pliny (A.D. 23-79), who called it stibium. Muslim alchemist Abu Musa Jabir Ibn Hayyan (c. 721-c. 815) probably first called it antimony?anti ("not") and monos ("alone"). The name comes from the fact that antimony does not occur alone in nature.
Alchemists used secret codes to write about much of their work, so modern scholars do not know a great deal about how antimony was used. The first detailed reports about antimony were published in 1707 when French chemist Nicolas Lemery (1645-1715) published his famous book, Treatise on Antimony.
Physical Properties
Antimony is a silvery-white, shiny element that looks like a metal. It has a scaly surface and is hard and brittle like a nonmetal. It can also be prepared as a black powder with a shiny brilliance to it.
The melting point of antimony is 630oC (1,170oC) and its boiling point is 1,635oC (2,980oF). It is a relatively soft material that can be scratched by glass. Its density is 6.68 grams per cubic centimeter.
Chemical Properties
Antimony is a moderately active element. It does not combine with oxygen in the air at room temperature. It also does not react with cold water or with most cold acids. It does dissolve in some hot acids, however, and in aqua regia. Aqua regia is a mixture of hydrochloric and nitric acids. It often reacts with materials that do not react with either acid separately.
Occurrence in Nature
Antimony is rarely found in its native (as an element) state. Instead, it usually occurs as a compound. The most common minerals of antimony are stibnite, tetrahedrite, bournonite, boulangerite, and jamesonite. In most of these minerals, antimony is combined with sulfur to produce some form of antimony sulfide (Sb2S3).
The largest producers of antimony are China, Russia, Bolivia, South Africa, and Kyrgyzstan, in that order. The United States produces antimony as a by-product at only one silver mine in Idaho.
The abundance of antimony is estimated to be about 0.2 parts per million, placing it in the bottom fifth among the chemical elements found in the Earth's crust. It is more abundant than silver or mercury, but less abundant than iodine.
Isotopes
There are two naturally occurring isotopes of antimony, antimony- 121 and antimony-123. Isotopes are two or more forms of an element. Isotopes differ from each other according to their mass number. The number written to the right of the element's name is the mass number. The mass number represents the number of protons plus neutrons in the nucleus of an atom of the element. The number of protons determines the element, but the number of neutrons in the atom of any one element can vary. Each variation is an isotope.
About 20 radioactive isotopes of antimony are also known. A radioactive isotope is one that breaks apart and gives off some form of radiation. Radioactive isotopes are produced when very small particles are fired at atoms. These particles stick in the atoms and make them radioactive.
Two of antimony's radioactive isotopes are used commercially as tracers. These isotopes are antimony-124 and antimony- 125. A tracer is an isotope injected into a living or non-living system. The movement of the isotope can then be followed as it moves through the system. For example, a small amount of antimony-124 could be injected into an oil pipeline. The presence of the isotope can be detected by means of an instrument held above the pipeline. The radiation given off by the isotope causes a light to flash or a sound to occur in the instrument. The movement of the isotope through the pipeline can be followed in this way. If the pipeline has a leak, the tracer will escape from it. Its movement through the soil can be detected.
Extraction
Antimony can be recovered from stibnite with hot iron:
2Fe + Sb2S3 -> Fe2S3 + 2Sb
About half the antimony produced in the United States is recycled from old lead storage batteries used in cars and trucks.
Uses
Antimony is used to make alloys with a number of different metals. An alloy is made by melting and mixing two or more metals. The properties of the mixture are different than those of the individual metals. One of the most common of these alloys is one made with lead. Lead-antimony alloys are used for solder, ammunition, fishing tackle, covering for electrical cables, alloys that melt at low temperatures, and batteries. The manufacture of lead storage batteries, like the ones used in cars and trucks, account for about one-fifth of all the antimony used each year. A small amount of antimony is also used in making transistors, which are found in such consumer electrical devices as computer games, pocket calculators, and portable stereos. A transistor is a solid-state (using special properties of solids, rather than electron tubes) electronic device used to control the flow of an electric current.
Other minor uses of antimony include the manufacture of glass and ceramics and the production of plastics. In glass and ceramics, a small amount of antimony insures that the final product will be clear and colorless. In the production of plastics, antimony is used as a catalyst. A catalyst is a substance used to speed up or slow down a chemical reaction. The catalyst does not undergo any change itself during the reaction.
Antimony Compounds
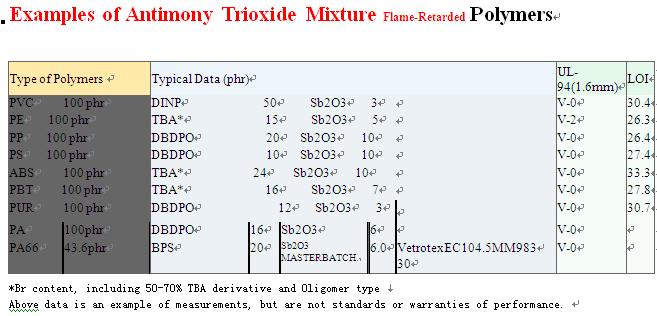
The most important use of antimony is in making compounds used in the manufacture of flame-retardant materials. Slightly more than half of all antimony goes to this use. These include antimony oxychloride (SbOCl), antimony pentoxide (Sb2O5), antimony trichloride (SbCl3), and antimony trioxide (Sb2O3). These compounds are sprayed on or added to a fabric to make it flame retardant.
Health Effects
Antimony and its compounds are dangerous to human health. In low levels, these materials can irritate the eyes and lungs. They may also cause stomach pain, diarrhea, vomiting, and stomach ulcers. At higher doses, antimony and its compounds can cause lung, heart, liver, and kidney damage. At very high doses, they can cause death.
Words to Know
Alchemy a kind of pre-science that existed from about 500 B.C. to about the end of the 16th century
Alloy a mixture of two or more metals with properties different from those of the individual metals
Aqua regia a mixture of hydrochloric and nitric acids that often reacts with materials that do not react with either acid separately
Catalyst a substance used to speed up or slow down a chemical reaction without undergoing any change itself
Isotopes two or more forms of an element that differ from each other according to their mass number
Metalloid an element that has characteristics of both metals and non-metals
Periodic table a chart that shows how the chemical elements are related to each other
Radioactive isotope an isotope that breaks apart and gives off some form of radiation
Solder an alloy that can be melted and then used to join two metals to each other
Toxic poisonous
Antimony is a chemical element in the periodic table that has the symbol Sb (L. Stibium) and atomic number 51. A metalloid, antimony has four allotropic forms. The stable form of antimony is a blue-white metal. Yellow and black antimony are unstable non-metals. Antimony is used in flame-proofing, paints, ceramics, enamels, a wide variety of alloys, electronics, and rubber.
Notable characteristics
Antimony in its elemental form is a silvery white, brittle, fusible, crystalline solid that exhibits poor electrical and heat conductivity properties and vaporizes at low temperatures. A metalloid, antimony resembles a metal in its appearance and physical properties, but does not chemically react as a metal. It is also attacked by oxidizing acids and halogens. Antimony and some of its alloys expand on cooling.
Estimates of the abundance of antimony in the Earth's crust range from 0.2 to 0.5 ppm. Antimony is geochemically categorized as a chalcophile, occurring with sulfur and the heavy metals lead, copper, and silver.
Applications
Antimony is increasingly being used in the semiconductor industry in the production of diodes, infrared detectors, and Hall-effect devices. As an alloy, this semi-metal greatly increases lead's hardness and mechanical strength. The most important use of antimony metal is as a hardener in lead for storage batteries. Other uses;
Batteries,
antifriction alloys,
type metal,
small arms and tracer bullets,
cable sheathing,
matches,
medicines,
plumbing ("lead-free" solder contains 5% Sb),
main and big-end bearings in internal combustion engines (as alloy).
used in the past to treat Schistosomiasis; nowadays Praziquantel is universally used.
Antimony compounds in the form of oxides, sulfides, sodium antimonate, and antimony trichloride are used in the making of flame-proofing compounds, ceramic enamels, glass, paints, and pottery. Antimony trioxide is the most important of the antimony compounds and is primarily used in flame-retardant formulations. These flame-retardant applications include such markets as children's clothing, toys, aircraft and automobile seat covers. Also, antimony sulfide is one of the ingredients of a modern match.
History
Antimony was recognized in antiquity (3000 BC or earlier) in various compounds, and it was prized for its fine casting qualities.
According to the history of metallurgy the first description of the procedure to isolate the antimony is in the Italian book "De la pirotechnia" of 1540 of Vannoccio Biringuccio. This book precedes the more famous Latin book "De re metallica" of 1556 of Agricola, although the latter has been often incorrectly considered the discoverer of the metallic antimony.
According to the traditional history of western alchemy the metallic antimony was previously (with respect to Biringuccio) described by the Prior Basilius Valentinus in the Latin manuscript "Currus Triumphalis Antimonii" of about 1450, published, in the English translation "The triumphal chariot of antimony", only in 1604 by Johann Thölde (1565-1614). The marvellous finding of all of the Valentinus' manuscripts, as in the alchemical tales, is fully described by Jean-Jacques Manget in his "Bibliotheca chemica curiosa" (1702): these manuscripts remained more than one century enclosed in a pillar of the St. Peter's Abbey, at Erfurt, until the pillar was miraculously shattered by a thunderbolt. Many authors consider Basilius Valentinus as a mythological personage: the most authoritative of them is Leibniz (1646-1716), that declared to be sure, after a careful search, that the Prior Valentinus did not ever exist in the Abbey of Erfurt, but was only a pseudonym, probably just of Thölde himself, that badly translated and merged materials of various origins.
According to the traditional history of Middle Eastern alchemy, the pure antimony was well known to Geber, sometimes called "the Father of Chemistry", in the 8th century. Here there is still an open controversy: Marcellin Berthelot, who translated a number of Geber's books, stated that the antimony is never quoted in them, but other authors claim that Berthelot translated only some of the less important books, while the more interesting ones (some of them perhaps well describing the antimony) are not yet translated, and their content is completely unknown.
The origin of the name "antimony" is not clear; the term may come from the Greek words "anti" and "monos", which approximately means "opposed to solitude" as it was thought never to exist in its pure form, or from the Pharaonic expression "Antos Amun", which could be translated as "bloom of the god Amun".
Alchemical symbol for antimonyThe natural sulfide of antimony, stibnite, was known and used in Biblical times as medicine and as a cosmetic. Stibnite is still used in some developing countries as medicine. Antimony has been used for the treatment of schistosomiasis. Antimony attaches itself to sulfur atoms in certain enzymes which are used by both the parasite and human host. Small doses can kill the parasite without causing damage to the patient. Antimony and its compounds are used in several veterinary preparations like Anthiomaline or Lithium antimony thiomalate, which is used as a skin conditioner in ruminants. Antimony has a nourishing or conditioning effect on keratinized tissues, at least in animals. Tartar emetic is another antimony preparation which is used as an anti-schistosomal drug.
The relationship between antimony's modern name and its symbol is complex; the Coptic name for the cosmetic powder antimony sulfide was borrowed by the Greeks, which was in turn borrowed by Latin, resulting in stibium. The chemical pioneer Jöns Jakob Berzelius used an abbreviation of this name for antimony in his writings, and his usage became the standard symbol.
Treatments chiefly involving antimony have been called antimonials.
Sources
Native massive antimony with oxidation productsEven though this element is not abundant, it is found in over 100 mineral species. Antimony is sometimes found native, but more frequently it is found in the sulfide stibnite (Sb2S3) which is the predominant ore mineral. Commercial forms of antimony are generally ingots, broken pieces, granules, and cast cake. Other forms are powder, shot, and single crystals.
Country Tonnes % of total
People's Republic of China 126 000 81.5
Russia 12 000 7.8
South Africa 5 023 3.3
Tajikistan 3 480 2.3
Bolivia 2 430 1.6
Top 5 148 933 96.4
Total world 154 538 100.0
Chiffres de 2003, métal contenue dans les minerais et concentrés, source : L'état du monde 2005
The largest mine in China is Xikuangshan mine in Hunan Province.
See also Antimonide minerals, Antimonate minerals.
Precautions
Antimony and many of its compounds are toxic. Clinically, antimony poisoning is very similar to arsenic poisoning. In small doses, antimony causes headache, dizziness, and depression. Such small doses have in the past been reported in some acidic fruit drinks. The acidic nature of the drink is sufficient to dissolve small amounts of antimony oxide contained in the packaging of the drink; modern manufacturing methods prevent this occurrence. Larger doses cause violent and frequent vomiting, and will lead to death in few days. Very large doses will cause violent vomiting, causing the poison to be expelled from the body before any harm is done.
See also arsenic poisoning.









0 comment:
Post a Comment