BEIJING (Asian Metal) 30 Jul 07 - Magnesium price keeps stable at around USD2,700-2,720/t with some sources insisting on selling at around USD2,750-2,800/t as high freight from China to Europe is still weighing on the market.
According to a source who offered at around USD2,800-2,820/t this week, many factors are making magnesium price high in Europe, despite the weak demand.
"I am sure consumers will come to accept at least price of around USD2,750-2,800/t in warehouse Rotterdam because they are aware of what is happening in the market, high freight, difficult to get a container etc. "
Another European trader who concluded deal this week at around USD2,750/t, shared the same view that suppliers in European market are insisting on around USD2,750-2,800/t in warehouse Rotterdam as the high freight keeps putting pressure on the price.
"I got offers from China at around USD2,590-2,600/t FOB China this week and freight has increased quite high," said the source. "But the market is still weak here in Europe with quotations in warehouse reaching USD2,800/t."
Many participants are not satisfied with the situation in magnesium market as demand is not increasing but price shows signs of increasing.
**********************
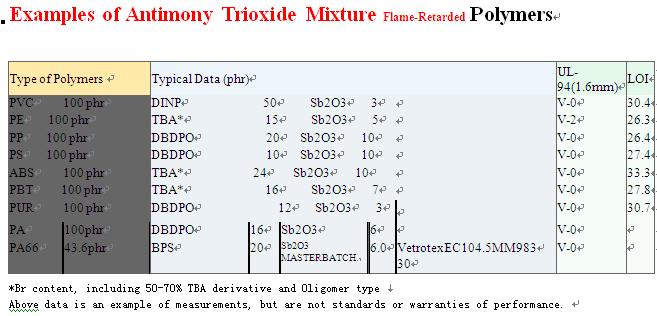
DONGGUAN JIEFU FLAME-RETARDED MATERIALS CO.,LTD
Sam Xu
Tel: 86-755-83474911
Fax: 86-755-83474980
Mobile:13929211059
E-mail: xubiao_1996(at)hotmail.com samjiefu(at)gmail.com
Add: jiefu industrial park shuiping industrail district dalang town dongguan GD,P.R.C
blog:http://antimony-trioxide.blogspot.com
website:http://www.jiefu.com
...
read more